Physical Changes
S5P1 Students will verify that an object is the sum of its parts.
a. Demonstrate that the mass of an object is equal to the sum of its parts by manipulating and measuring different objects made of various parts.
b. Investigate how common items have parts that are too small to be seen without magnification.
S5P2 Students will explain the difference between a physical change and a chemical change.
a. Investigate physical changes by separating mixtures and manipulating (cutting, tearing, folding) paper to demonstrate examples of physical change.
b. Recognize that the changes in state of water (water vapor/steam, liquid, ice) are due to temperature differences and are examples of physical change.
a. Demonstrate that the mass of an object is equal to the sum of its parts by manipulating and measuring different objects made of various parts.
b. Investigate how common items have parts that are too small to be seen without magnification.
S5P2 Students will explain the difference between a physical change and a chemical change.
a. Investigate physical changes by separating mixtures and manipulating (cutting, tearing, folding) paper to demonstrate examples of physical change.
b. Recognize that the changes in state of water (water vapor/steam, liquid, ice) are due to temperature differences and are examples of physical change.
Changing MatterEverything is made of matter. The smallest possible pieces of different types of matter are called atoms. Atoms join together in various combinations to make molecules. For example, water is made of H2O, which is two hydrogen atoms and one oxygen atom. Matter frequently changes. Some changes are physical changes, which are merely changing the way something looks or feels, and sometimes the matter changes into an entirely new matter (chemical change). The list below shows some common examples of each type of change.
Visit StudyJams to see a video about physical and chemical changes in matter.
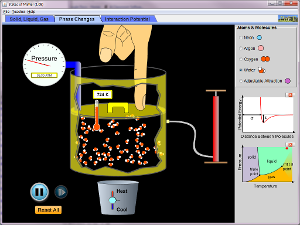
Three States of MatterThere are three states of matter: solid, liquid and gas. When the temperature changes the molecules react and cause the material to changes state. For example, water changes to ice when the temperature is lowered to 32 degrees Fahrenheit. Water boils and turns into water vapor when it is heated to 212 degrees Fahrenheit. Visit StudyJams for a more complete video explanation and quiz.
The simulation linked below may not work on all devices or computers. It is however, a fantastic simulation which allows you to see how varying temperatures affect the molecules in various substances. |
Mass, Volume, Density & WeightYou can visit StudyJams for a more detailed explanation of mass, volume, density and weight. I'll give you a basic overview here:
Mixtures and Solutions |